ACS Publications Industry Webinar
Behind the Failures of Suzuki–Miyaura Coupling Scale-Up: A Real-World Case Study
On Demand
Gain Practical Insights from an Industry Expert on the Challenges of Scaling up API Manufacturing
Process chemists in the pharmaceutical industry constantly face the challenge of delivering active pharmaceutical ingredients (APIs) on tight timelines, often requiring the rapid scale-up of reactions from laboratory flask to multi-thousand-liter manufacturing reactors.
The Suzuki-Miyaura coupling reaction is widely utilized in API manufacturing. Although most companies have developed proprietary know-how for scaling up this important reaction, failures can still occur due to the intrinsic complexities associated with palladium-catalyzed processes, such as sensitivity to air, temperature, mixing, and others, as well as the significant challenges of removing residual palladium from the final product.
In this webinar, guest speaker Dr. Yamamoto from Takeda Pharmaceuticals shares a real-world case study of an unexpected failure during API manufacturing involving the Suzuki-Miyaura coupling. The discussion focuses on the unforeseen root causes that emerged during scale-up and how the team addressed and overcame these challenges.
This webinar is ideal for professionals involved in process development, scale-up, and API manufacturing, as well as anyone working in process chemistry or related fields. Register to watch on demand.
Register to watch the webinar.
SPEAKER INFORMATION

Yuhei Yamamoto, Ph.D.
Senior Director, Head of Process Chemistry, Takeda Shonan (TSHO), Synthetic Molecule Process Development, Pharmaceutical Sciences, Takeda Pharmaceutical Company Limited
Dr. Yuhei Yamamoto is Senior Director and Head of Process Chemistry at Takeda Shonan (TSHO), a key site within Takeda’s global Synthetic Molecule Process Development organization. With over 20 years of experience in pharmaceutical sciences—primarily in process chemistry—Dr. Yamamoto has a proven track record in organic synthesis, process research, GMP manufacturing, regulatory documentation, analytical development, technology transfer, and cross-functional leadership.
Dr. Yamamoto has authored approximately 20 scientific publications and has built strong academic and industry collaborations globally. Before joining Takeda, he worked at Banyu Pharmaceutical (now MSD), a subsidiary of Merck & Co., where he gained international experience in locations including Rahway, NJ. He received his B.S., M.S., and Ph.D. from Kyoto University and further conducted research as a visiting scientist at The University of Chicago under Professor Hisashi Yamamoto.
RESEARCH SPOTLIGHT
Org. Process Res. Dev. 2025, 29, 9, 2339–2345
https://doi.org/10.1021/acs.oprd.5c00207
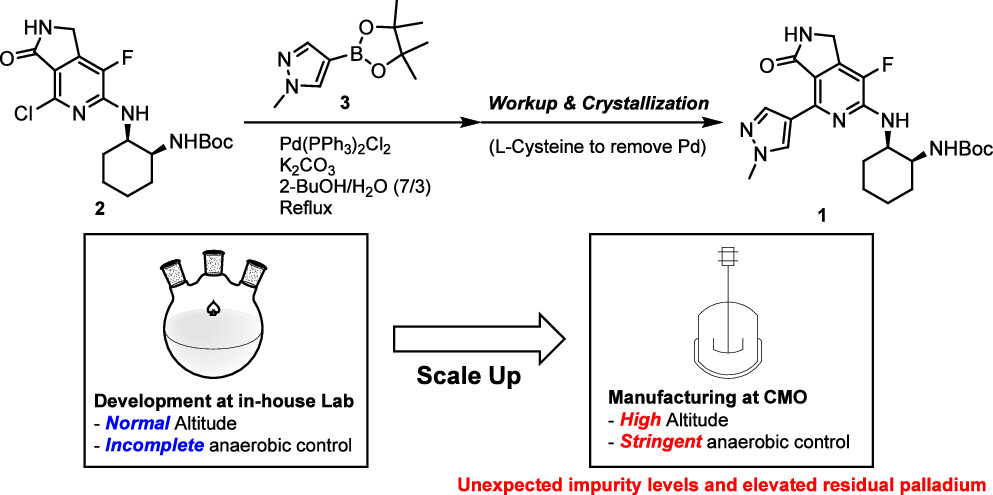
This study presents the lessons learned from scaling up the Suzuki–Miyaura coupling reaction to a 50 kg scale. The reaction conditions were optimized at 89–90 °C, corresponding to the boiling point of the solvent system (2-BuOH/H2O, 7/3). Manufacturing at this scale was conducted at a contract manufacturing organization (CMO) situated at high altitude, requiring the use of a pressure vessel to maintain the internal temperature within the desired range. Reaction, workup, and crystallization processes were performed under stringent anaerobic conditions to prevent adverse events typically associated with palladium-catalyzed reactions...
Org. Process Res. Dev. 2022, 26, 10, 2882–2893
https://doi.org/10.1021/acs.oprd.2c00215
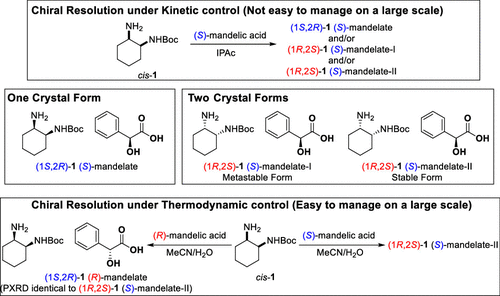
A practical method for isolating tert-butyl [(1S,2R)-2-aminocyclohexyl]carbamate ((1S,2R)-1) in its pure form was investigated. Two different patents from different groups have shown that (1S,2R)-1 can be isolated in the pure form using either (S)-mandelic acid or (R)-mandelic acid as a chiral resolving reagent. Our investigation revealed that both (1S,2R)-1 and (1R,2S)-1 form crystals with (S)-mandelic acid, and the latter salt has two crystal forms with different physicochemical properties including the solubilities and the crystal form transformation. The detailed physicochemical studies on the three crystal forms revealed that the patented processes proceed under kinetic control, which is not easy to manage on a large scale. Our process optimization based on the three crystal form behaviors led to the establishment of a process for manufacturing either (1S,2R)-1 (R)-mandelate or (1R,2S)-1 (S)-mandelate from commercially available cis-1,2-diaminocyclohexane. The newly developed process is considered to proceed under thermodynamic control and has been demonstrated on a large scale.
Copyright © 2026 | American Chemical Society | 1155 Sixteenth Street NW | Washington, DC 20036